Redox Signaling – A Revolutionary Approach to Improving Cellular Health
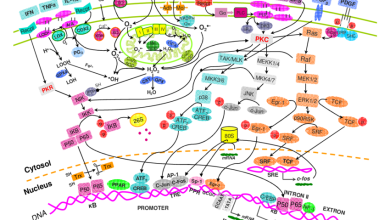
Inflammation, oxidative stress, and other pathological conditions induce an elevated intracellular redox state. This can trigger physiological signaling through reversible oxidation of specific cysteines in target proteins.
This redox signaling depends on the kinetics and location of the source and target, with site-specificity determined by the pKa of the protein thiol. This is an important finding for the development of next-generation redox medicine.
The homeostatic balance between the rate of ROS generation and enzymatic/non-enzymatic antioxidant systems that scavenge ROS is essential for healthy cell function. This balance is disrupted during oxidative stress when ROS accumulation exceeds the capacity of the antioxidant system. The resulting redox imbalance triggers signaling pathways that induce a specific physiological change in cellular function (also referred to as redox-dependent signaling).
Oxidative signals can be released directly from mitochondria to the cytoplasm or derived from the interaction of diffusible nonradical ROS with redox-sensitive transducer proteins by reversible covalent modification such as carbonylation, cysteine oxidation, or methionine sulfoxidation. In vivo studies have shown that these signals can influence various biological processes, such as cell cycle control, cellular differentiation, neuronal cell loss during neurodegenerative diseases, and aging.
One use for ROS is as a signaling molecule by competing with O2 at the respiratory complex IV in mitochondria and slowing the respiration rate or via S-nitrosation of protein thiol groups to modulate their activity independent of H2O. In addition, ROS can interact with the iron sulfide centers of proteins such as aconitase to regulate its activity directly.
Despite the significant role of redox signaling in cellular physiology, the development of therapeutics targeting redox signaling has proven challenging. This is mainly due to the uncontrolled side effects exhibited by most first-line therapies, such as radiotherapy and anti-oxidative agents. Therefore, we need to understand better the mechanism of action of redox signaling to develop effective, safe, and precise interventions targeting different redox-related targets and redox-sensitive molecules.
What is Redox Signaling?
Redox Signaling is a system of signal transduction that encodes cellular processes. It involves reversible electron transfer reactions incorporating free radicals and related species, redox-active metals, or reductive equivalents, such as the hydrogen atom donated by reduced glutathione or thiol proteins. The revolutionary approach of redox signaling in improving cellular health prompts individuals to explore various sources of information and insights, such as ASEA reviews, to comprehend better the potential benefits and effects of redox-based solutions on overall well-being and physiological function.
Reversible electron transfer reactions involving cysteine residues are the core of redox signaling. Cysteine is an essential amino acid that can be modified through S-nitrosylation, sulfenylation, and disulfide bond formation to form various functional groups, including nitrosothiols and disulfides (SNO, SNO2).
These chemical species activate downstream cellular processes such as transcription, proliferation, or apoptosis through interaction with redox-sensitive mechanisms that bind and modulate specific target proteins, e.g., kinases, phosphatases, and ion channels. For example, the cytosolic copper-zinc superoxide dismutase (SOD) isoforms of the endoplasmic reticulum (ER) promote ROS production in a redox signaling pathway that is required for adaptive immunity.
How Does Redox Signaling Work?
While oxidants like ROOH and 4-hydroxy-2-nonenal (HNE) are generally known as damaging oxidants, they can also act as signaling molecules. The conditions required are based on hydroperoxides’ reaction kinetics and the signaling proteins’ cellular location. The specificity of signaling with these oxidants involves the presence of protein cysteines in microenvironments where their pKa is low enough to dissociate from the thiolate, allowing the donation of a proton and forming an addition product.
These products can then potentiate agonist-induced calcium signals or induce cell apoptosis. Induction of antioxidant genes, such as nitric oxide synthases, can also be achieved by these reactions.
The cytosolic copper-zinc superoxide dismutase (CuZnSOD), mitochondrial manganese superoxide dismutase (MnSOD), and extracellular superoxide dismutase (ecSOD) are the first line of defense against the production of ROS in healthy cells. These enzymes convert oxidative stress-generated H2O2 to the non-harmful hydroxyl radical, HO. The free HO can then activate nuclear enhancer Nrf2 to promote the expression of many antioxidant genes, thus protecting against oxidative stress.
How Can Redox Signaling Help You?
As they say in real estate, “location, location, location.” This is particularly true when it comes to redox signaling. Steep gradients and competition for reaction with electrophiles require that the target of the signaling protein be close to its source.
For example, nicotinamide adenine dinucleotide phosphate (NADP+)-derived reactive oxygen species (ROS) generated by NADPH oxidase act as kindling to trigger secondary and tertiary ROS-generating enzymes such as uncoupled eNOS, xanthine oxidase, and mitochondrial complex I. These enzymes create superoxide anions and hydrogen peroxide, which can damage DNA and lipids and cause cell death.
The nuclear factor erythrocyte-responsive element binding protein (ARE) and its promoter tightly regulate this cellular self-defense mechanism. Redox-sensitive activation results in the transcription of antioxidant stress genes such as catalase, superoxide dismutase, glutathione S-transferases, phase-I detoxification enzymes (glutathione reductase, NAD(P)H quinone oxidoreductase and heme oxygenase-1), which protect against oxidative damage. The resulting redox imbalance is balanced by the enzymatic production of reduced glutathione (GSH), which can also repair lipid peroxidation and adducts.